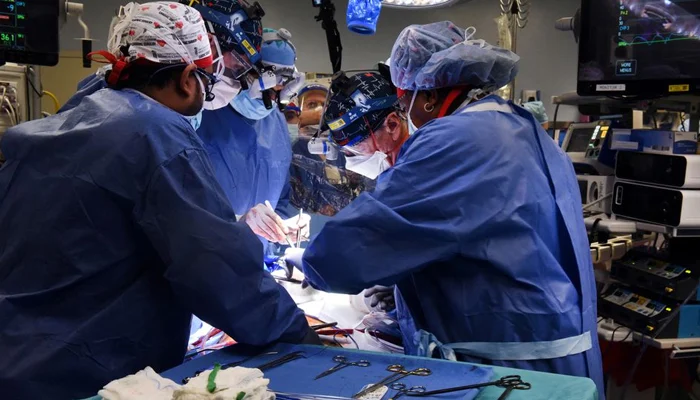
According to health experts, a genetically modified pig kidney that was transplanted into a human patient in the US exhibited remarkable resilience, surviving for over a month without any signs of malfunction. This development is part of the ongoing exploration into the feasibility of successful cross-species organ transplantation.
Robert Montgomery, who serves as the director of the NYU Langone Transplant Institute, shared with reporters that they had achieved a significant milestone: “We have observed a genetically-edited pig kidney thriving inside a human body for more than a month.”
Montgomery spearheaded the pioneering effort of performing the first-ever transplantation of a genetically modified pig kidney into a human recipient in September 2021, followed by a similar procedure in November of the same year. Expressing confidence in the progress made, he remarked, “At this stage, there is a highly compelling narrative that should instill confidence in initiating preliminary studies… involving living human subjects.”
Subsequent to these initial endeavors, a few additional instances have been recorded, although these experiments lasted for only two to three days.
In contrast to previous attempts involving multiple genetic alterations, the most recent procedure focused on a single genetic modification. Specifically, the team targeted the gene linked to “hyperacute rejection,” a phenomenon that would typically occur within minutes of integrating an animal organ into a human circulatory system.
By effectively disabling the gene responsible for producing a biomolecule called alpha-gal, which is susceptible to attack by human antibodies, the researchers at NYU Langone managed to prevent immediate rejection.
Montgomery elaborated on their findings, stating, “We have accumulated further evidence indicating that, especially in the context of kidneys, eliminating the gene that triggers hyperacute rejection, in combination with clinically approved immunosuppressive medications, could potentially lead to the successful management of transplants in humans over the long term.”
Additionally, the team incorporated the thymus gland of the pig, located in the neck region and responsible for educating the immune system, into the outer layer of the transplanted kidney. This incorporation facilitated the host’s immune cells in recognizing the pig’s cells as part of its own, thereby thwarting delayed rejection.
In the process, the patient’s own kidneys were removed, and a single pig kidney was transplanted, promptly initiating urine production. Close monitoring indicated that the waste product creatinine was at optimal levels and no signs of rejection were evident.
Notably, the absence of porcine cytomegalovirus, a virus associated with potential organ failure, has been a significant observation. The research team intends to continue surveillance for another month to gather more data.
In January 2022, surgeons at the University of Maryland Medical School performed the world’s first pig-to-human transplant on a living patient. Unfortunately, the patient passed away two months later, with the presence of porcine cytomegalovirus in the organ identified as a contributing factor.
The source of the donor pig was a herd affiliated with the Virginia-based biotech company Revivicor. This particular herd had received approval from the Food and Drug Administration (FDA) as a meat source suitable for individuals with hypersensitivity to the alpha-gal molecule, an allergy triggered by tick bites.
The pigs used in these endeavors are bred, not cloned, which facilitates a more scalable process. Current endeavors remain focused on pigs due to their advantageous characteristics as potential donors, including their organ size, rapid growth rate, large litters, and their existing role as a food source.