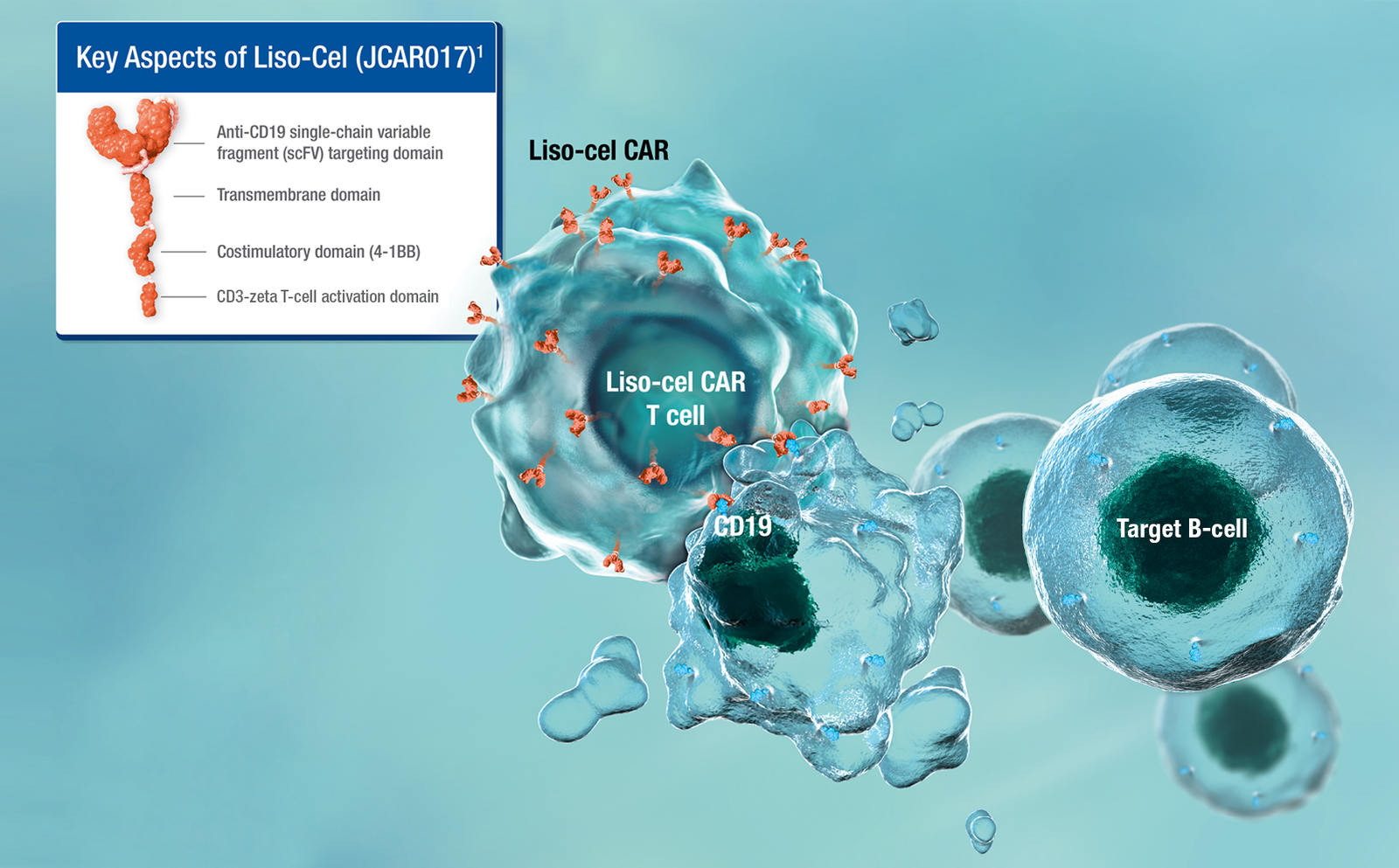
On Wednesday, the U.S. health regulator granted approval for the expanded utilization of Bristol Myers Squibb’s cancer cell therapy, Breyanzi, for the treatment of adults afflicted with follicular lymphoma, a type of blood cancer that has either recurred or shown resistance to previous treatments.
This decision by the Food and Drug Administration (FDA) marks Breyanzi’s fourth approval, extending its application to patients who have undergone two or more prior lines of therapy.
Bryan Campbell, Bristol Myers’ head of commercial cell therapy, highlighted the significance of this approval, noting the therapy’s potential for lasting remission through a single infusion and its manageable safety profile, facilitating administration and monitoring across a growing number of certified treatment centers in the U.S.
Initially approved in 2021 as a second-line treatment for large B-cell lymphoma, Breyanzi falls under the category of chimeric antigen receptor (CAR) T-cell therapies, which modify T-cells to target cancer cells.
Follicular lymphoma, a prevalent form of non-Hodgkin lymphoma, typically affects individuals aged 50 years and older, manifesting as malignant cancer cells within the lymph system.
According to U.S. government data, the annual rate of new cases of non-Hodgkin lymphoma stands at 18.6 per 100,000 men and women.
Patients with follicular lymphoma commonly experience periods of remission and relapse, with treatment becoming progressively challenging after each recurrence.
Clinical data from a mid-stage study revealed promising outcomes, with Breyanzi achieving complete cancer elimination in 94% of patients who had undergone at least two prior treatments. Additionally, 97% of patients exhibited signs of cancer disappearance or shrinkage following treatment with Bristol’s therapy.
Bristol anticipates a substantial market expansion for Breyanzi with this additional approval, potentially doubling its reach.
The company is actively bolstering its manufacturing capacity for CAR-T therapies, including Abecma and Breyanzi, in response to escalating demand.
Other treatment alternatives include cell therapies such as Novartis’ Kymriah and Gilead Sciences’ Yescarta, underscoring the evolving landscape of cancer treatment options.