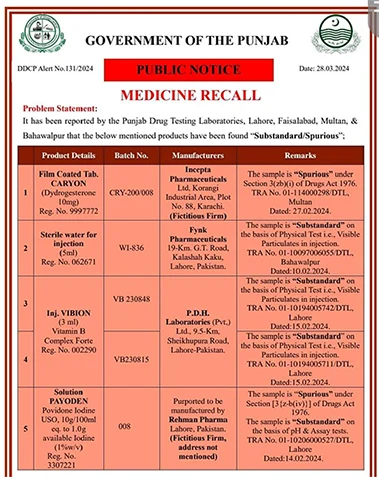
A recent analysis conducted by the Drug Testing Laboratory Punjab has unveiled alarming findings regarding the quality of medicines being sold in Lahore. The report reveals that a staggering 11 medicines available in the market are substandard and counterfeit, posing grave risks to public health and safety.
Among the identified counterfeit drugs are treatments for infertility and wound dressings, raising serious concerns about the efficacy and safety of these essential medical products. Additionally, the analysis detected particles of various materials in four different injections, intended for surgeries and epileptic seizures, further highlighting the extent of the issue.
One particularly troubling discovery was the excessive presence of ethynyl glycol in four different brands of cough and cold syrups, all of which were deemed substandard. This chemical, when present in quantities exceeding prescribed limits, can pose serious health hazards to consumers, underscoring the urgent need for regulatory intervention.
The counterfeit drugs identified in the analysis include Carrion Tablet by Incepta Pharmaceuticals and Piodine Solution by Rehman Pharma, while the substandard medications comprise Water for Injection by Fynk Pharmaceuticals, Vibyan Injection by PDH Laboratories, Ketosive Injection by Medici Pharmaceuticals, Epigran Injection by Atco Laboratories, and several syrups produced by various pharmaceutical companies.
In response to these alarming findings, the Directorate of Drug Control Punjab has issued immediate directives for the withdrawal of the substandard batches of medicines from the market. Additionally, stringent action has been initiated against the pharmaceutical companies responsible for manufacturing these defective products.
The investigation also revealed that nine of the identified substandard medicines were being manufactured in Lahore, while two were produced in Karachi, highlighting the need for comprehensive oversight and regulation across the pharmaceutical industry nationwide. The widespread prevalence of substandard and counterfeit drugs underscores the urgent need for robust quality control measures and vigilant enforcement of regulatory standards to safeguard public health.
The implications of the discovery extend beyond the immediate health risks posed to consumers. The proliferation of substandard and fake medicines undermines trust in the healthcare system, erodes confidence in pharmaceutical products, and jeopardizes the credibility of the industry as a whole. Moreover, it exposes vulnerable populations to unnecessary harm and exacerbates existing healthcare disparities.
Efforts to address this pressing issue must involve collaboration between regulatory authorities, pharmaceutical companies, healthcare providers, and consumers. Enhancing surveillance mechanisms, strengthening quality control protocols, and implementing stringent penalties for non-compliance are essential steps towards combating the menace of substandard and counterfeit drugs.
Furthermore, public awareness campaigns and educational initiatives are crucial for empowering consumers to make informed choices and recognize the signs of counterfeit or substandard medicines. By fostering a culture of accountability and transparency, stakeholders can work together to mitigate the risks associated with substandard medications and uphold the integrity of the pharmaceutical industry.
As regulatory agencies continue to crack down on illicit practices and ensure compliance with quality standards, it is imperative that the health and safety of the public remain paramount. The revelations uncovered by the Drug Testing Laboratory Punjab serve as a wake-up call for concerted action to eliminate the scourge of substandard and fake medicines, safeguarding the well-being of communities across Lahore and beyond.