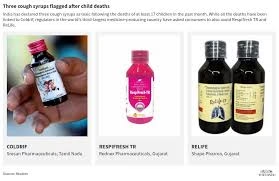
Authorities in India’s southern state of Tamil Nadu have cancelled the manufacturing licenses of Sresan Pharmaceuticals, the company linked to the deaths of at least 19 children in Madhya Pradesh, officials confirmed on Monday. The move comes as investigators conduct money-laundering raids at multiple company sites.
According to state authorities, the children died after consuming a cough syrup produced by the Tamil Nadu-based firm. Laboratory tests earlier this month revealed the medicine contained nearly 500 times the permissible limit of diethylene glycol, a highly toxic industrial solvent known to cause kidney failure and death.
“The manufacturing licenses of Sresan Pharmaceuticals have been completely cancelled, and the company has been shut down,”
the Tamil Nadu government said in an official statement.
The Enforcement Directorate (ED), India’s financial crime-fighting agency, has launched searches across seven locations in Chennai, including the homes of senior officials in the state’s drug control department, amid allegations of money laundering tied to the company’s operations.
Sresan Pharmaceuticals’ owner, G. Ranganathan, who was arrested last week, has not responded to media inquiries. The ED has yet to issue an official statement regarding the ongoing probe.
Wider Concerns Over India’s Pharmaceutical Safety
India, often referred to as the “pharmacy of the world”, supplies 40% of generic drugs used in the United States and over 90% of medicines in many African nations. However, this incident has once again raised global concerns over drug quality and regulatory enforcement in the country’s vast pharmaceutical sector.
The tragedy mirrors earlier scandals in 2023, when Indian-made cough syrups were linked to the deaths of children in Cameroon, The Gambia, and Uzbekistan.
Under Indian law, manufacturers must test all raw materials and finished products, while exports of cough syrups have required additional government lab testing since 2023. Despite these rules, the World Health Organization (WHO) recently warned of a persistent “regulatory gap” in the screening of domestically sold medicinal syrups.