A groundbreaking bioengineering discovery promises to repair damaged bones without the adverse side effects common in current treatments, offering hope for improved patient outcomes. This innovative approach could transform treatments for individuals with severe skeletal injuries or cancer patients experiencing bone loss by promoting bone tissue regrowth.
Scientists in Scotland have pioneered a new method to harness the potent healing properties of “growth factors” – naturally-occurring molecules critical to the body’s regenerative processes. These molecules are fundamental in developmental biology, guiding the body’s growth from infancy to adulthood and playing a vital role in the healing process after injuries by orchestrating complex tissue repair mechanisms.
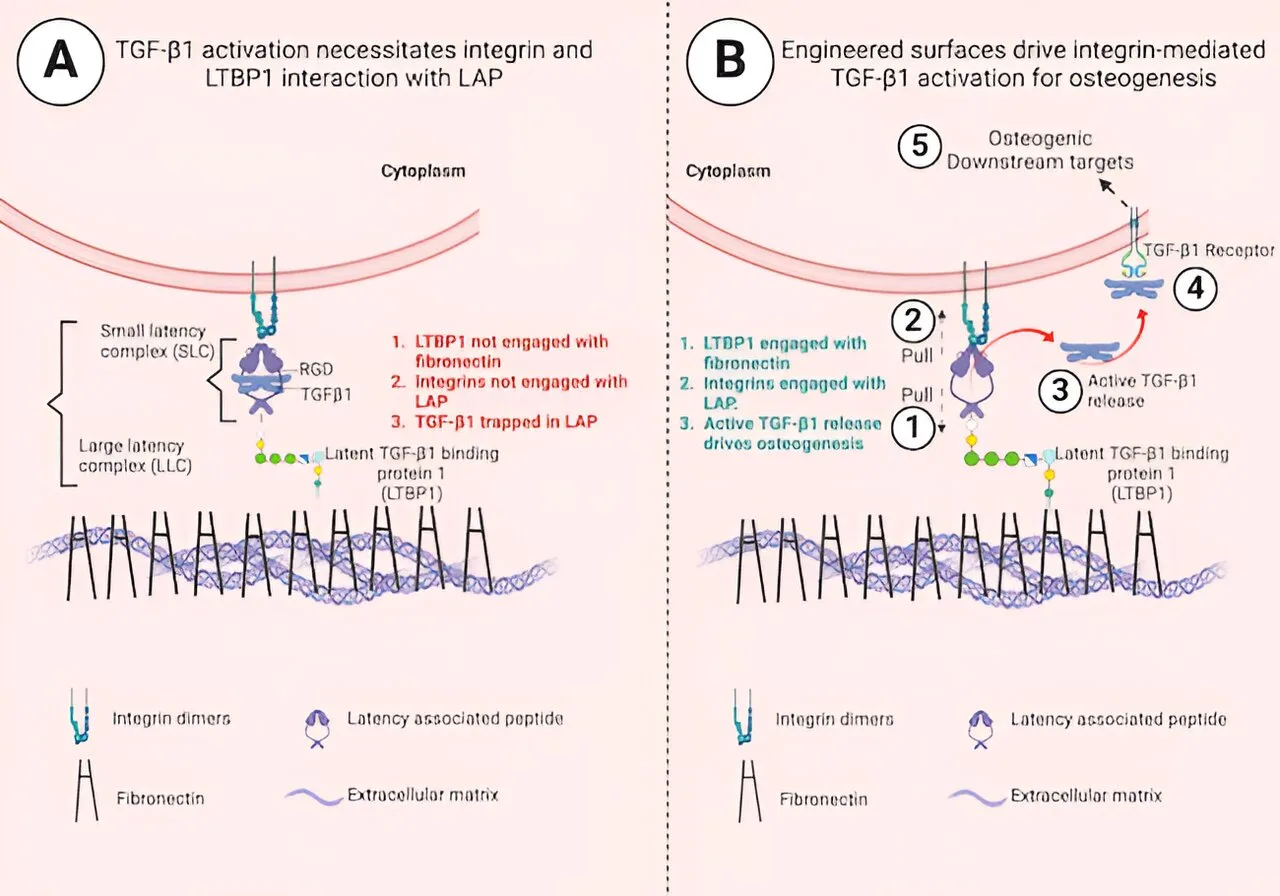
The research team, led by the University of Glasgow, has detailed their significant breakthrough. They utilized an inexpensive polymer called poly(ethyl acrylate) (PEA) to create a surgical implant for bone defect sites. The implant’s unique surface properties enable it to capture the body’s inactive growth factors, ensuring they activate only at the required location.
Dr. Udesh Dhawan, Research Fellow at the University of Glasgow’s James Watt School of Engineering, explained, “The biological processes underlying this study have been understood for more than two decades, but this is the first time they’ve been harnessed to produce this regenerative effect. Delivering immobilized proteins directly to the treatment site in this way provides much more control over how growth factors become active and initiate the healing process. It also operates at much lower concentrations than previous treatments, further minimizing the risk of unwanted bone growth beyond the intended site.”
Previous research by the team demonstrated that PEA interacts with fibronectin, a protein abundant in the human body that promotes cell adhesion and growth, to form nanoscale networks on its surface. This network formation alters the shape of fibronectin, exposing certain amino acids in the molecule, thereby enhancing its ability to aid in tissue regeneration.
This breakthrough signifies a promising advancement in bone repair technology, potentially leading to more effective and safer treatments for patients suffering from severe bone damage or loss.