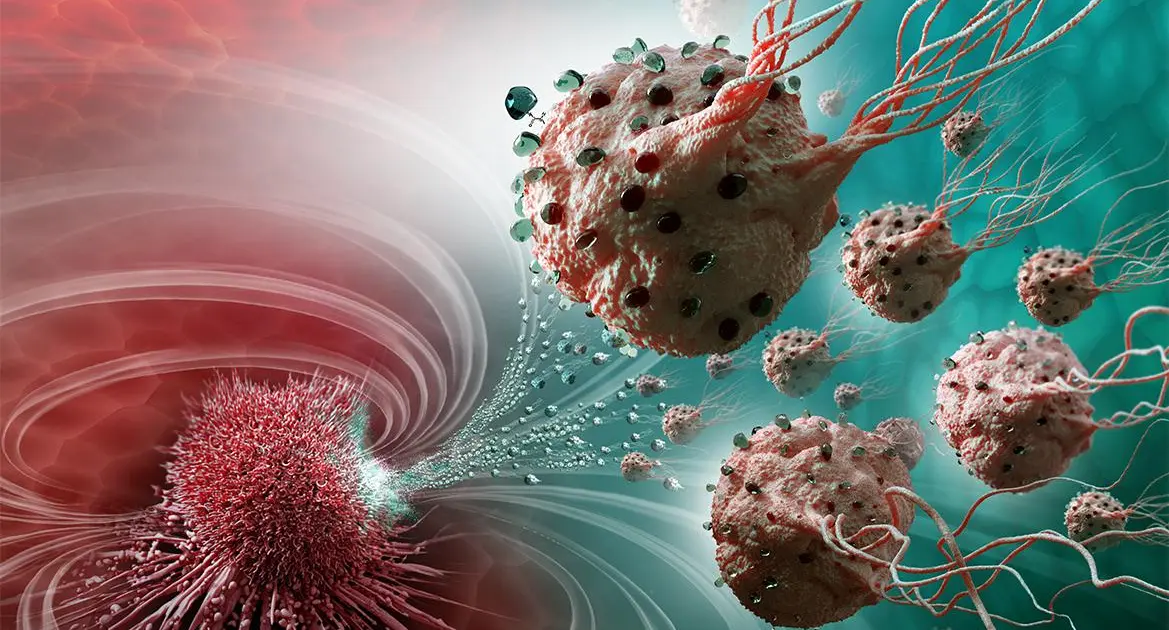
In a promising development for cancer treatment, researchers have created nanobots that have demonstrated the ability to kill cancer cells in mice.
Previously, researchers at Karolinska Institutet developed structures that organize death receptors on cell surfaces, inducing cell death. These structures are made of six peptides (amino acid chains) arranged in a hexagonal pattern.
Death receptors are like switches on cell surfaces that, when activated by specific signals like tumor necrosis factor (TNF), trigger apoptosis, a process leading to cell self-destruction. This mechanism helps regulate cell survival and death.
The hexagonal nanopattern of peptides acts as a powerful weapon, but administering it as a drug could indiscriminately kill cells throughout the body, posing significant risks. To address this, researchers have encapsulated the weapon within a DNA nanostructure.
Using DNA origami, a technique that creates nanoscale structures out of DNA, researchers can design small shapes with high precision, placing DNA pieces exactly where needed and attaching proteins to form precise patterns and structures at the molecular level.
They applied this technology to develop a “kill switch” that activates under specific conditions. “We have managed to hide the weapon in such a way that it can only be exposed in the environment found in and around a solid tumor,” explained Björn Högberg, a professor at the Department of Medical Biochemistry and Biophysics, Karolinska Institutet.
The nanorobot’s weapon is activated by the acidic microenvironment, characterized by a low pH, typically found around cancer cells. In test tube analyses, the peptide weapon remained concealed at a normal pH of 7.4 but became exposed and lethal when the pH dropped to 6.5.
When tested in animals with breast cancer tumors, the nanorobot led to a 70 percent decrease in tumor growth compared to mice that received an inactive version of the nanorobot.
“We now need to investigate whether this works in more advanced cancer models that more closely resemble the real human disease,” said Yang Wang, a researcher at Karolinska Institutet.
The team must also assess potential side effects before human testing, such as the risk of killing healthy cells outside of tumors if certain conditions are met. “These results are an early proof of concept and are in no way a real treatment today. Our plan to investigate this includes moving to more realistic animal models that incorporate, for example, mice with humanized death receptors,” Högberg told Interesting Engineering.
Additionally, researchers plan to enhance the nanorobot’s targeting capabilities by attaching proteins or peptides to its surface that bind specifically to certain types of cancer.
Promising Breakthrough in Cancer Treatment: Nanobots Developed to Target and Kill Cancer Cells
In a promising development for cancer treatment, researchers have created nanobots that have demonstrated the ability to kill cancer cells in mice.
Previously, researchers at Karolinska Institutet developed structures that organize death receptors on cell surfaces, inducing cell death. These structures are made of six peptides (amino acid chains) arranged in a hexagonal pattern.
Death receptors are like switches on cell surfaces that, when activated by specific signals like tumor necrosis factor (TNF), trigger apoptosis, a process leading to cell self-destruction. This mechanism helps regulate cell survival and death.
The hexagonal nanopattern of peptides acts as a powerful weapon, but administering it as a drug could indiscriminately kill cells throughout the body, posing significant risks. To address this, researchers have encapsulated the weapon within a DNA nanostructure.
Using DNA origami, a technique that creates nanoscale structures out of DNA, researchers can design small shapes with high precision, placing DNA pieces exactly where needed and attaching proteins to form precise patterns and structures at the molecular level.
They applied this technology to develop a “kill switch” that activates under specific conditions. “We have managed to hide the weapon in such a way that it can only be exposed in the environment found in and around a solid tumor,” explained Björn Högberg, a professor at the Department of Medical Biochemistry and Biophysics, Karolinska Institutet.
The nanorobot’s weapon is activated by the acidic microenvironment, characterized by a low pH, typically found around cancer cells. In test tube analyses, the peptide weapon remained concealed at a normal pH of 7.4 but became exposed and lethal when the pH dropped to 6.5.
When tested in animals with breast cancer tumors, the nanorobot led to a 70 percent decrease in tumor growth compared to mice that received an inactive version of the nanorobot.
“We now need to investigate whether this works in more advanced cancer models that more closely resemble the real human disease,” said Yang Wang, a researcher at Karolinska Institutet.
The team must also assess potential side effects before human testing, such as the risk of killing healthy cells outside of tumors if certain conditions are met. “These results are an early proof of concept and are in no way a real treatment today. Our plan to investigate this includes moving to more realistic animal models that incorporate, for example, mice with humanized death receptors,” Högberg told Interesting Engineering.
Additionally, researchers plan to enhance the nanorobot’s targeting capabilities by attaching proteins or peptides to its surface that bind specifically to certain types of cancer.