The Directorate of Drugs Control, Punjab, has issued a public notice ordering the immediate recall of certain medicines declared substandard and adulterated, directing manufacturers to withdraw the affected batches from the market without delay.
According to the alert, the Drug Testing Laboratories Punjab declared the listed medicines as substandard and adulterated. Following these findings, the Provincial Quality Control Board approved the recall of the products from across the province.
Among the recalled medicines is Injection Neudex 1mL (Dexamethasone sodium phosphate equivalent to Dexamethasone phosphate 4mg/mL), Registration No. 042943. Batches DX063 and DX079 with expiry dates of October 2027, and batch DX080 expiring in November 2027, were declared adulterated by the Drug Testing Laboratories Punjab.
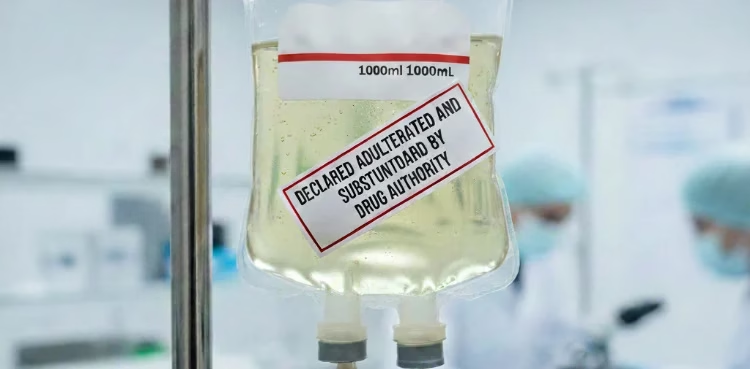
The Directorate has also recalled Infusion Zeesol H 1000mL (Ringer Lactate), containing Calcium Chloride, Potassium Chloride, Sodium Chloride, and Sodium Lactate. Batch No. 2503281, with an expiry date of February 2030, was declared substandard after failing sterility tests.
The manufacturers of the affected products have been instructed to immediately recall the specified batches and submit a complete distribution trail. They have also been directed to upload the “Recall Assessment Form” on the Drug Regulatory Authority of Pakistan (DRAP) website, conduct a detailed root cause analysis, and develop a Corrective and Preventive Action (CAPA) plan.
In addition, the provincial Drug Control Directorate has advised healthcare professionals and regulators, including pharmacies, medical stores, wholesalers, distributors, and healthcare facilities, to stop dispensing these products immediately. All stakeholders have been instructed to report current stock levels and consumption records to their respective area Drug Inspectors or Drug Inspector Distributors.
All field formations across Punjab have been directed to enhance surveillance within supply chains and take strict regulatory action in accordance with the Drug Act 1976 and the DRAP Act 2012.