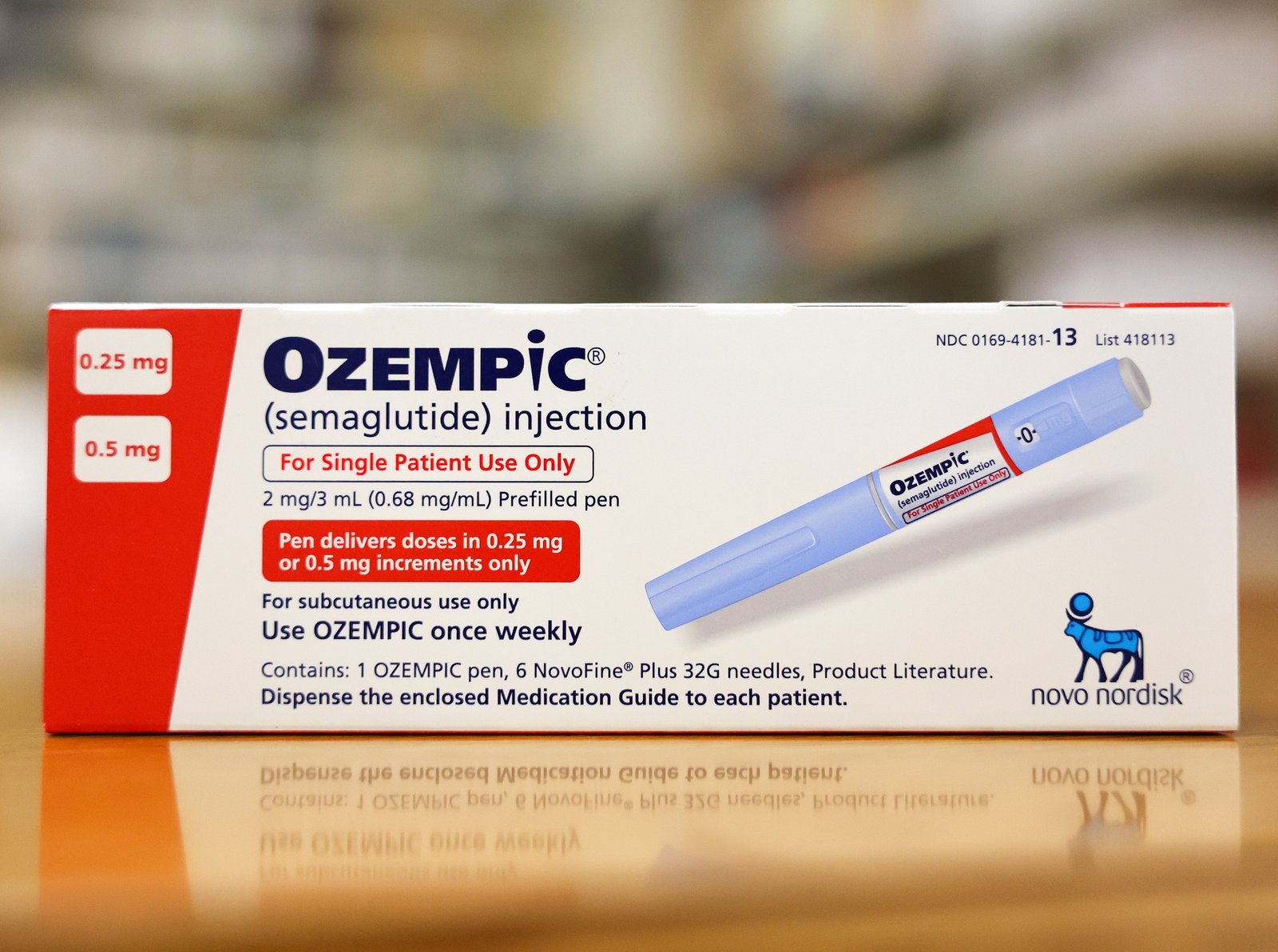
Patients taking Novo Nordisk’s Ozempic and Wegovy, as well as Eli Lilly’s Mounjaro, have reported experiencing suicidal thoughts, prompting concerns and investigations into the safety of these popular drugs used to treat diabetes and obesity. All three medications belong to the class of GLP-1 receptor agonists, which are designed to slow digestion and reduce hunger.
A Reuters investigation revealed that the U.S. Food and Drug Administration (FDA) has received 265 reports of suicidal thoughts or behaviors in patients taking these drugs or similar medications since 2010. Of these reports, 36 describe deaths by suicide or suspected suicide. The FDA is currently evaluating these reports and will determine whether further action, such as warning labels, is necessary after a thorough review.
While the adverse-event reports serve as a warning system for the medical community, they do not establish a definitive causal link between the drugs and the reported side effects. However, these reports have raised concerns, especially when patients without a history of depression suddenly experience suicidal thoughts after starting or increasing their dosage of these medications.
The Reuters review is the first comprehensive examination of FDA adverse-event reports related to suicidal thinking associated with GLP-1 drugs. The investigation also involved obtaining 113 detailed narratives for individual incidents from the 265 reports in the FDA database. The experiences of the patients who shared their stories with Reuters have not been previously reported.
Novo Nordisk has submitted 180 of the 265 reports to the FDA regarding suicidal thinking or behaviors. The company maintains confidence in the benefit-risk profile of its products and cites its own safety monitoring, which has not found a “causal association” between the drugs and thoughts of self-harm.
Eli Lilly, the maker of Mounjaro, is collaborating with regulators on potential safety issues associated with its drug. Dr. Erick Turner, a former FDA medical officer, emphasized the importance of cases where patients without a history of depression experience suicidal thoughts suddenly after starting or increasing their dose of medication. Such cases raise credibility concerns regarding safety signals.
The scrutiny of GLP-1 drugs has intensified, with European regulators launching an investigation into suicide risk from these medications in July. Health agencies in the UK and Canada are also reviewing the suicide risk associated with these drugs.
While GLP-1 drugs like Wegovy have shown effectiveness in weight loss, healthcare providers and regulators are monitoring for previously undocumented dangers as more patients begin using them. The FDA may consider strengthening warnings or issuing black-box warnings if evidence suggests an increased suicide risk.
Patients who spoke with Reuters shared their experiences of sudden and intense suicidal thoughts while taking these drugs. Some patients were unaware that their experiences were being reported to the FDA, highlighting the need for better communication between patients and healthcare providers regarding potential side effects.
While the FDA continues to evaluate these reports, concerns remain about the safety of GLP-1 drugs, which have gained popularity in treating diabetes and obesity. Balancing the benefits and potential risks of these medications is a priority for healthcare professionals and regulators.