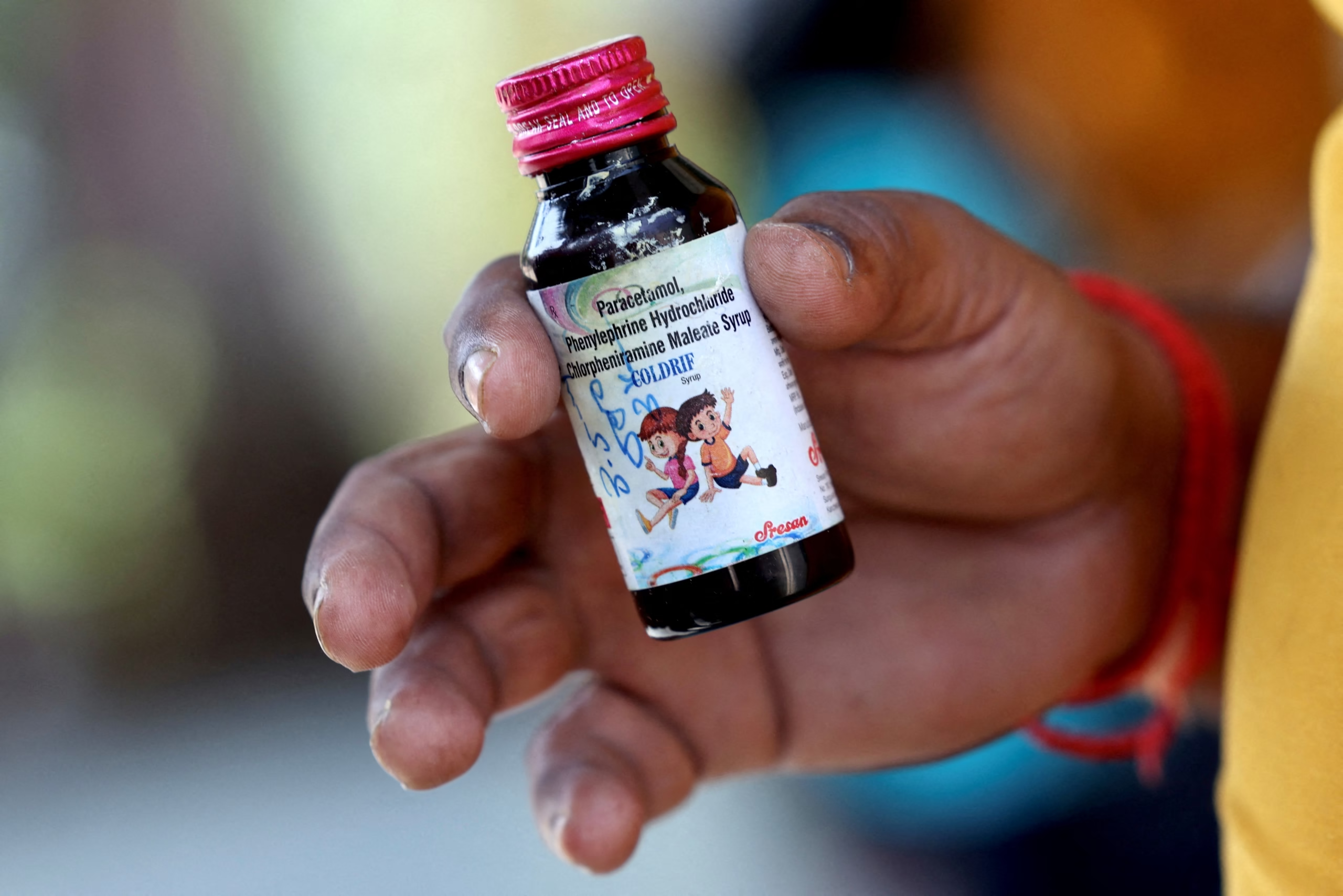
India has issued a nationwide warning against two additional cough syrup brands — Respifresh and RELIFE — after 17 children under the age of five died from consuming medicines contaminated with a toxic chemical, officials confirmed on Wednesday.
The children, who died over the past month, were all linked to Coldrif syrup, which was found to contain dangerously high levels of diethylene glycol — nearly 500 times above the permissible limit. The drug was banned on October 2 after tests confirmed contamination.
Health alerts issued by Gujarat and other states described diethylene glycol as “a toxic chemical that can cause serious poisoning, including kidney failure, neurological complications, and even death, especially among children.”
WHO Seeks Clarity on Exports
The World Health Organization (WHO) told Reuters it is seeking clarification from New Delhi on whether any of the toxic syrups were exported to other countries.
According to a government document, Coldrif, made by Sresan Pharmaceutical Manufacturer, was sold only within India, but Respifresh and RELIFE were distributed in other Indian states. It remains unclear whether they were exported abroad.
The WHO said it will decide whether to issue a Global Medical Products Alert once it receives confirmation from Indian authorities. The agency reiterated its guidance against the use of cough and cold medicines for children.
Factory Inspections and Legal Action
India’s Drug Controller General, Rajeev Raghuvanshi, said inspections revealed serious lapses at factories producing the contaminated syrups. Some manufacturers failed to test every batch of ingredients and finished products, as required by law.
In an advisory dated October 7, Raghuvanshi said inspections were carried out at firms previously flagged for substandard drugs. Although he did not name the companies, officials confirmed that Shape Pharma (maker of RELIFE) and Rednex Pharmaceuticals (maker of Respifresh) were among those inspected.
Samples from both companies were declared “not of standard quality”, leading to an immediate suspension of production and distribution. Neither company responded to media inquiries.
Meanwhile, Sresan Pharmaceutical’s factory in Tamil Nadu was found abandoned and sealed by authorities. Police are investigating the company for manslaughter, and central regulators have recommended revoking its manufacturing licence. At the site, inspectors found discarded syrup bottles and burnt medicines, with a strong chemical odor hanging in the air.
Industry-Wide Impact
The contamination scandal echoes earlier tragedies in Gambia, Uzbekistan, and Cameroon, where Indian-made cough syrups containing ethylene and diethylene glycol caused the deaths of over 140 children since 2022.
India’s $50 billion pharmaceutical industry — the world’s third-largest by volume — supplies 40% of generic medicines used in the United States and more than 90% of medicines in many African nations.
Following the recent deaths, authorities have launched inspections at 19 manufacturing units across six states to prevent further incidents and restore confidence in India’s drug safety standards.