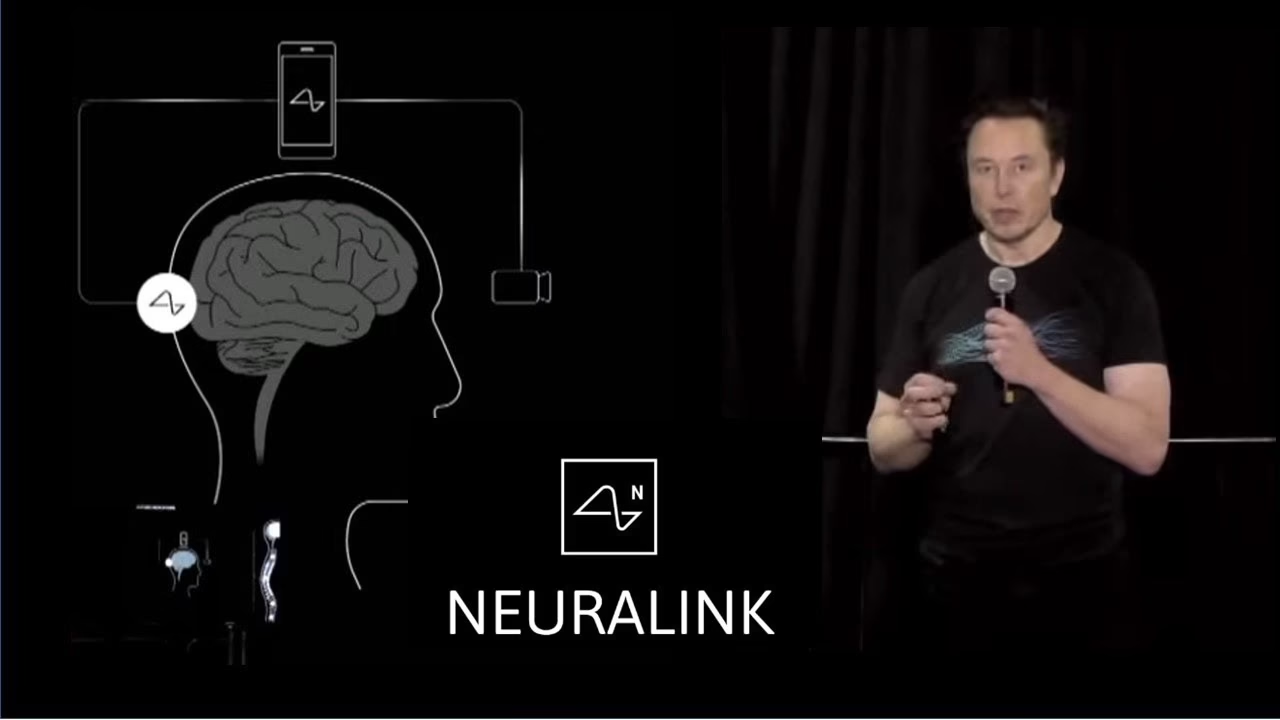
Neuralink, Elon Musk’s brain-computer interface company, announced on Thursday that it has received the U.S. Food and Drug Administration’s “breakthrough device” designation for a new implant designed to restore communication in people with severe speech impairments.
The device aims to assist individuals affected by neurological conditions such as amyotrophic lateral sclerosis (ALS), stroke, cerebral palsy, spinal cord injuries, and multiple sclerosis, according to a company post on X (formerly Twitter).
The FDA’s breakthrough devices program is designed to accelerate the development and review process for technologies that offer significant advantages over existing options for patients with life-threatening or debilitating conditions.
Neither the FDA nor Neuralink responded immediately to media requests for further comment.