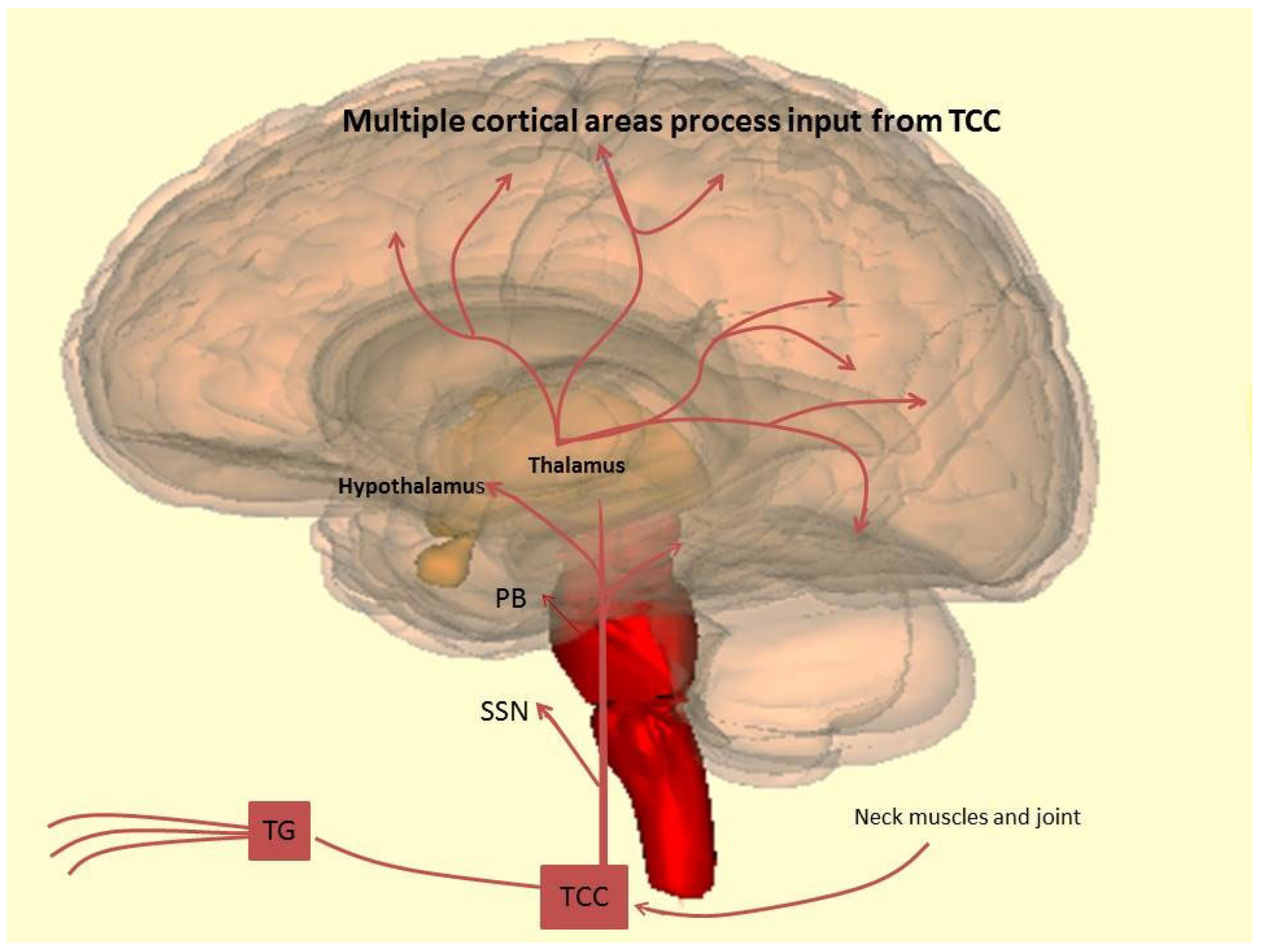
A recent study published in Neurology, the medical journal of the American Academy of Neurology, suggests that ubrogepant, a CGRP inhibitor, may significantly enhance migraine treatment.
The research indicates that taking ubrogepant at the onset of migraine symptoms could help individuals maintain their daily activities with minimal disruption.
The study involved 518 participants who experienced migraines 2 to 8 times per month and tested the efficacy of ubrogepant in treating early migraine signs.
Participants were divided into two groups: one took a placebo initially and ubrogepant during a second migraine attack, while the other group received ubrogepant first and a placebo later.
Participants rated their activity limitations on a scale from 0 (not limited) to 4 (extremely limited) in a daily diary.
The findings revealed that 65% of those who took ubrogepant felt “not at all limited” or “a little limited” 24 hours later, compared to 48% of those who took the placebo.
Two hours after taking the medication, ubrogepant users were 73% more likely to report no disability than those who received the placebo.
These results suggest that early administration of ubrogepant could be effective in managing migraines, allowing sufferers to continue their daily routines with less interruption.
However, the study noted a potential limitation: the reliance on self-reported diary entries, which could introduce inaccuracies.